Manipulating the mouse genome
Mice carrying genetic alterations can be made by manipulating embryonic stem (ES) cells.
ES cells are usually derived from the inner cell mass of blastocyst stage embryos. Pre-implantation embryos are explanted into culture and attach to the substratum. The cells of the blastocyst spread over the feeder layer and cell lines can be isolated from the epiblast component and can be maintained in culture through multiple passages. Under appropriate culture conditions these cell lines will maintain a normal karyotype and posses the ability to differentiate into a wide spectrum of different cell types in vitro including neurons, glia, skeletal, cardiac and smooth muscle, endoderm and haematopoietic precursors.
If ES cells are microinjected into the blastocoel cavity of a host blastocyst they will combine with the inner cell mass component and contribute to the developing embryo. Offspring have somatic tissue composed of both ES cell derived cells and host blastocyst derived cells. These animals are termed chimeras and demonstrate the true pluripotentiality of the ES cells. Various markers can be used to demonstrate chimaerism. If the ES cell line is derived from a strain of mouse with different coat colour markers from the strain used to obtain the host blastocyst then hair pigmentation can be easily used to assess contribution. Most importantly, however, the chimaeras not only have somatic tissue contribution from the ES cells but also have germ cell contribution. The ability of ES cells to colonise the germ line of chimaeric animals and the fact that they can be maintained in culture for long periods provides a route to the generation of transgenic mice. Any genetic alteration introduced into the ES cells in culture can be incorporated into a chimaera and subsequently transmitted to their offspring.
Gene targeting
Mice carrying defined genetic alterations can be generated by gene targeting in embryonic stem cells. Gene targeting is defined as the genetic modification of an endogenous DNA sequence within a cell by homologous recombination with a DNA segment introduced into the cell. The first gene to be targeted in ES cells was the hypoxanthine phosphoribosyl transferase (hprt) gene. The hprt gene is located on the X chromosome and therefore is only present as one copy in male ES cells. Cells that have lost a functional hprt gene can be selected in 6-thioguanine, a toxic purine analogue that kills hprt+ cells. The ability to readily select for hprt– ES cells allowed the identification of ES clones that had undergone inactivation of the hprt gene. The demonstration that these hprt– ES lines could be repaired through homologous recombination and still colonise the germline of chimaeras demonstrated that gene targeting could be used to generate mice theoretically carrying a specific mutation at any genetic locus.
Targeted disruption of most genes other than hprt, however, has the problem that selection cannot be used to identify targeted clones. The targeting DNA must, therefore, contain a selectable marker to allow cells that incorporate the DNA into their genome to be selected from the cells that do not take up or integrate the DNA. In addition, only a small proportion of the transformed cells have undergone homologous recombination. Thus, various strategies have been developed to enrich for targeted clones. The most widely used method is known as the positive/negative selection method. The input DNA into the ES cells carries both a positive selection marker (eg. neor gene selected with G418) and a 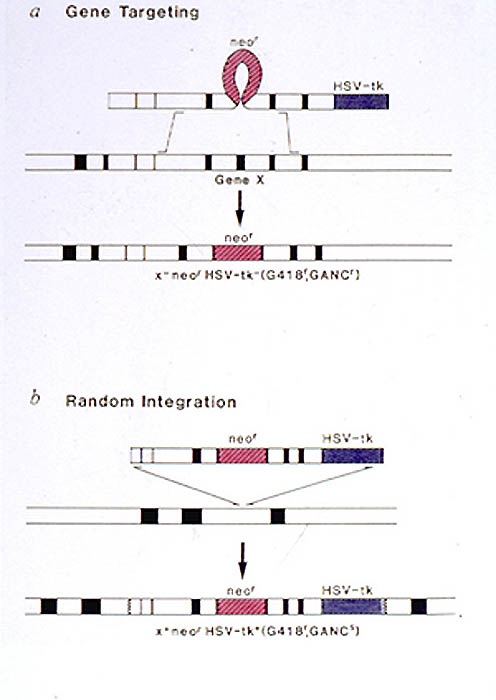 negative selection gene (eg. HSV-tk selected against with gancyclovir). For transfected cells to survive in the presence of both G418 and gancyclovir they must acquire the neor gene but not the tk gene. Random integrations will not result in loss of the tk gene and these cells will die in the presence of gancyclovir. If homologous recombination occurs through a double cross over event, then the tk gene will be lost and these clones will grow in the presence of gancyclovir. This selection scheme generally gives about a 10-fold enrichment for targeted clones.
negative selection gene (eg. HSV-tk selected against with gancyclovir). For transfected cells to survive in the presence of both G418 and gancyclovir they must acquire the neor gene but not the tk gene. Random integrations will not result in loss of the tk gene and these cells will die in the presence of gancyclovir. If homologous recombination occurs through a double cross over event, then the tk gene will be lost and these clones will grow in the presence of gancyclovir. This selection scheme generally gives about a 10-fold enrichment for targeted clones.
Here is shown the TK positive/negative selection process. Cells die in gancyclovir-containing media when expressing the thymidine kinase gene product. Thus, selection with gancyclovir enhances the number of cells which have undergone a correct homologous recombination event, and therefore lost the tk portion of the targeting vector.
Cells die in gancyclovir-containing media when expressing the thymidine kinase gene product. Thus selection with gancyclovir enhances the number of cells which have undergone a correct homologous recombination event, which should not incorporate the tk portion of the targeting vector. The manipulation of genes at their endogenous chromosomal location and the ability to transmit these genetic alterations into mice are powerful means for addressing many biological questions. It is therefore not surprising that gene targeting in ES cells has become widely used in many areas of biology to elucidate the function of a gene in a whole animal environment and also to model human genetic disorders.
Gene trapping
Gene trapping in embryonic stem cells can be used to tag and mutate genes. The expression pattern of the mutated gene can be visualized by staining for activity of the LacZ gene product, to screen for trapped genes that are expressed at a particular development stage or in a specific tissue. We are currently characterising four gene traps that show restricted expression in the gonads and are establishing the role of these genes in gametogenesis.

