Submitted by Emily Rigby on Tue, 28/05/2024 - 12:37
A new paper titled “Mechanical stress combines with planar polarised patterning during metaphase to orient embryonic epithelial cell divisions” has been published in Development from Guy Blanchard, Elena Scarpa, Leila Muresan and Bénédicte Sanson.
A large body of work supports the Hertwig Rule, which states that the orientation of cell division follows the prior orientation of the cell shape during interphase. Most cells lose their shape and become rounded in mitosis, so how cells retain a memory of their shape has been a long-standing question in the field. The authors show that in the Drosophila embryonic epithelium, cell division does not rely on a memory of interphase cell shape. Instead, cell division orientation is set by a combination of embryonic patterning and mechanics during mitosis itself.
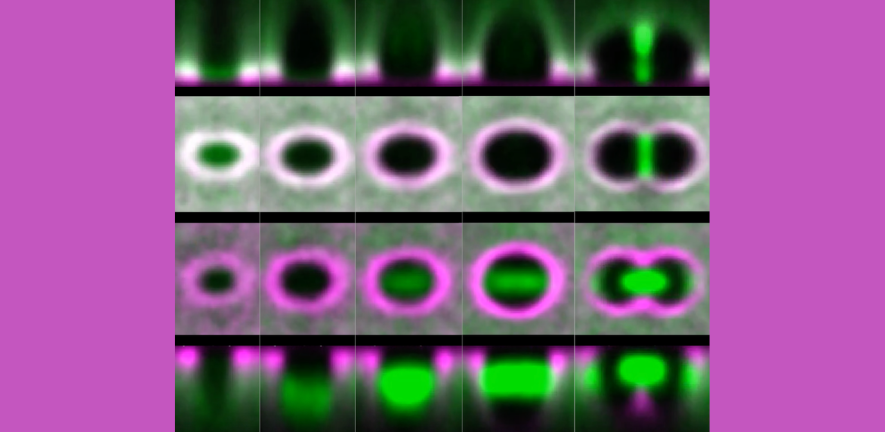
They developed bespoke computational image analysis methods to analyse hundreds of cell divisions in multiple embryo movies, tracking cell shapes and patterns of cellular protein fluorescence from interphase through mitosis. They used laser cuts at both cellular and tissue scales to interrogate the orientation of greatest stress.
The team found that cells maintained some elongation despite rounding during mitosis and that cell long axes could be re-oriented during metaphase by changes in the orientation of greatest stress across cells. Interestingly, the mitotic spindle is often longer than the cells planar short axis, forcing the spindle, and the subsequent division orientation, towards the new cell long axis.
On the other hand, it was discovered that when cells have isotropic, non-elongated shapes, they tend to orient their mitotic spindle towards anterior-posterior (AP) planar polarised cues (e.g. Myosin-II), a bias that is lost in an AP-patterning mutant.
Thus strong mechanical stress anisotropy leads to strong cell elongation which constrains the mitotic spindle along the cell long axis, whereas AP patterned cues will determine the division orientation in mechanically isotropic cells. In between, a compromise is reached between steric hindrance of the spindle and its attraction towards AP.
Dr Blanchard says "The Drosophila embryonic epithelium is an excellent model tissue for cell division not least because it is an immature epithelium without septate junctions or ECM. Active forces during cell division are therefore readily manifested as changes in cell shape in ways not seen in mature epithelia. There are also strong similarities with comparable vertebrate embryonic epithelia, in which AP-patterning is also known to orient cell divisions, but our results suggest that more work is needed to understand the influence of mechanics on division orientation in vertebrate epithelia."
For the full paper see https://doi.org/10.1242/dev.202862.
Example embryo movie of the ventrolateral embryonic trunk overlaid with tracked cell shapes (magenta) and tracked 3D spindles (green/red/blue).

