Research Title
Adaptive Regulation of Organ Size
Areas of interest
Regulation of organ size and body proportions
Limb and bone development
Catch-up growth
Inter-species chimeras
Join the Rosello-Diez Lab
We welcome applications from enthusiastic, highly motivated students and talented postdocs. If you are interested in joining the lab, send an email to Alberto Rosello-Diez outlining your research interests. Also include a CV with the contact details of at least two people who can provide a letter of reference.
Biography
During his career, Alberto has used chicken embryology and sophisticated mouse genetics to determine how individual cells collect and process internal and external information to make decisions, and how the combination of these individual decisions leads to robust and precise organogenesis. He uses the vertebrate limb as a model to address these questions.
Alberto received his BSc/MSc degrees in biochemistry and molecular biology from the University of Zaragoza, Spain. He then completed his PhD in 2011 in Molecular Biology (Autonomous University of Madrid) at the Spanish National Centre for Cardiovascular Research (Madrid, Spain), in the group of Dr. Miguel Torres. His PhD work, focused on the control of limb patterning by intrinsic and extrinsic mechanisms, was included in Gilbert’s Developmental Biology textbook. During his postdoc at the Memorial Sloan Kettering Cancer Center (New York, USA) with Dr Alex Joyner (2012-2018), he pioneered mouse genetic models of unilateral perturbations of limb growth to study the repair of developmental defects, finding that cell-non autonomous responses play a key role in this repair. In June 2018 he joined the Australian Regenerative Medicine Institute (Monash University, Melbourne) as a Group Leader, where his lab studied the organ-repair responses activated by developmental perturbations, with a focus on the long bones of the limbs. In June 2023 he joined the University of Cambridge as Associate Professor in Developmental Genetics, with a joint appointment between the Dept. of Physiology, Development and Neuroscience, and the Dept. of Genetics. A new line of work at Cambridge will use inter-species chimeras to study if/how the patterning, tempo and size of the limb are affected by the embryonic environment of a different species.
Research
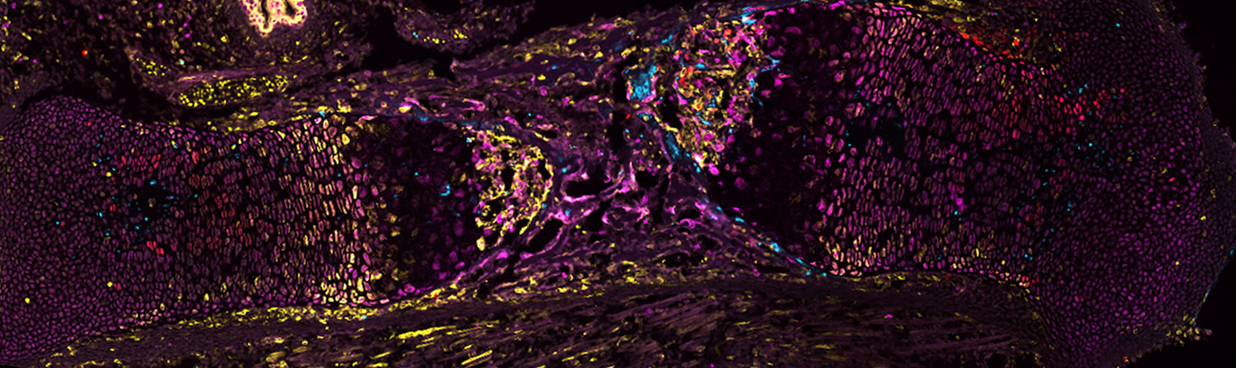
Did you ever wonder about how organs "know" how much they must grow in order to attain and maintain species-specific body proportions? And how this collective outcome emerges from the combination of individual cell behaviours? So do we! Our group uses sophisticated genetic models of perturbed limb development to study the local and systemic mechanisms by which growth perturbations are detected and compensated. We use mainly mice, and soon transgenic chicken, in which we combine classic morphometric and histological analyses with state-of-the-art imaging and 'omics' techniques to obtain a more holistic view of intra- and inter-organ cell communication during organ development and regeneration. While long bones in the limbs are our starting point, we also study how they crosstalk with other connective tissues and with distant organs. Bone elongation is driven by the growth plate (GP), a transient cartilage structure where cells called chondrocytes transition from resting (quiescent, stem cell-like) to proliferative and then hypertrophic. Hypertrophic chondrocytes either die or transdifferentiate into osteoblasts (bone-laying cells), so that the cartilage is replaced by bone. Bone growth is controlled by GP-intrinsic and GP-extrinsic factors, but the extent to which these interact is quite unknown. Especially important for regenerative medicine are skeletal stem cells (defined by their ability to give rise to cartilage, bone and marrow). Their location, identity and fate are actively investigated, and recent studies showed that bone repair and development use different pools of stem cells that could be targeted by distinct therapies. We are currently working on 3 main projects:
Cellular and molecular mechanisms of catch-up growth
One powerful approach to study the regulation of organ size is analysing how organs recover a normal growth trajectory after a developmental insult, which is known as catch-up growth. This project will address the mechanisms that trigger the compensatory response upon insult. On the signalling aspect, we hypothesise that this phenomenon involves feedforward control based on production of an alarm signal. We will determine the requirement of candidate alarm signals produced by insulted cells and the role of the subsequently activated signalling pathways. Moreover, we are analysing at the single-cell level the lineage and behaviours of key stem cell populations, comparing normal and catch-up growth scenarios. The goal is to find the foetal precursors of the long-lived cartilage progenitors, whose proliferative potential is what determines final bone size.
Identifying the sizostat (a thermostat for size)
When one of the two growth plates in a bone is damaged, the other one can partially compensate for its absence, suggesting that there is a target bone size for age, and that a feedback mechanism informs cartilage cells of bone size. We hypothesise that bone elongation progressively generates a mechanical signal that is transduced into a biochemical one, which in turn affects cartilage activity. We think that this mechanism works as a thermostat, such that there is an age-dependent threshold for the mechano-transduced signal, and when this signal approaches the threshold, growth is stalled, and vice versa. We are using sophisticated imaging and genetic tools to monitor and manipulate these mechanical and biochemical signals, with the long-term goal of being able to manipulate individual bone length at will.
Using inter-species chimeras to identify the determinants of limb size
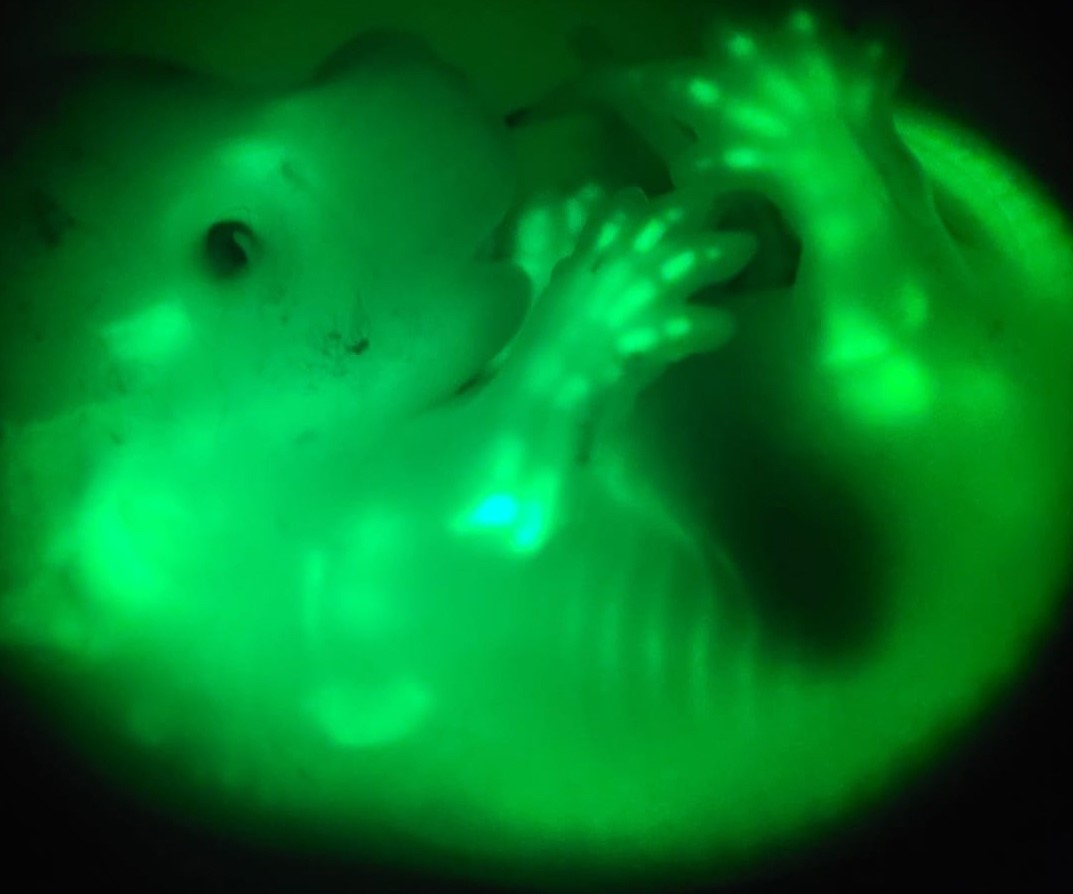
A classic approach to study limb size determination is to cross-graft limb primordia (a.k.a. buds) between close species that differ in size. Initial experiments suggested that the size of the donor limb was unaffected or only slightly modulated by the host. However, we and others showed that when the whole limb bud is taken, its own epithelium exerts a strong instructive influence, so that limb bud cells can only be reprogrammed by extrinsic host signals when they are taken from the undifferentiated limb region of young donors and isolated from the influence of the epithelium. Moreover, limb-bud cells lose their fate plasticity quite early in development, so that by the time the limb is big enough to be grafted, it might be too late for it to be reprogrammed by the signalling environment. Therefore, the question remains as to whether the outcome of the inter-species transplantation would be different if donor limb cells developed from the beginning within the environment of the host. In this project, we will generate for the first time chimeric animals in which stem cells of species that differ in size from the host are incorporated early on in the host embryo, giving rise to the limb connective tissues (bones, tendons, dermis, etc) in the context of the host’s signals. Detailed anatomical characterisation will assess whether mechanical loading, hormones and other extrinsic signals can modulate the genetically-encoded limb size and proportions. Gene expression and chromatin accessibility will be compared for the donor limb cells in their endogenous vs. the chimeric context, to uncover the key molecular events underlying this modulation.
For more information, please visit https://www.rosellodiezlab.com
Publications
Compensatory growth and recovery of cartilage cytoarchitecture after transient cell death in fetal mouse limbs. H'ng CH, Amarasinghe SL, Zhang B, Chang H, Qu X, Powell DR, Rosello-Diez A. Nat Commun. 2024 Apr 5;15(1):2940. doi: 10.1038/s41467-024-47311-7. PMID: 38580631
A collection of genetic mouse lines and related tools for inducible and reversible intersectional mis-expression. Ahmadzadeh E, Bayin NS, Qu X, Singh A, Madisen L, Stephen D, Zeng H, Joyner AL, Rosello-Diez A. Development. 2020 May 28;147(10):dev186650. doi: 10.1242/dev.186650. PMID: 32366677.
Cell-nonautonomous local and systemic responses to cell arrest enable long-bone catch-up growth in developing mice. Rosello-Diez A#, Madisen L, Bastide S, Zeng H, Joyner AL#. PLoS Biol. 2018 Jun 26;16(6):e2005086. doi: 10.1371/journal.pbio.2005086. PMID: 29944650. #Co-corresponding authors. Highlighted in https://www.nature.com/articles/d41586-018-05540-z
Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Rosello-Diez A, Arques CG, Delgado I, Giovinazzo G, Torres M. Development. 2014 Apr;141(7):1534-43. doi: 10.1242/dev.106831. PMID: 24598165.
Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Rosello-Diez A, et al. Science. 2011 May 27; 332(6033):1086-8. doi: 10.1126/science.1199489. PMID: 21617076.