The middle ear structures of birds, reptiles and frogs differ from those of mammals in that the tympanic membrane is coupled to the stapes, the only ear ossicle in these animals, by means of a cartilaginous structure called the extrastapes (the stapes and extrastapes of these animals are sometimes referred to as the "columella" and "extracolumella" respectively). Fishes lack a tympanic middle ear, but some species have Weberian ossicles to couple swimbladder vibrations to the sensory structures of the inner ear. Find out more by following the links below:
My research has largely concentrated on frog ears:
- My latest collaboration, with Dr. Sandra Goutte and an international team, has been to look at the hearing of pumpkin toadlets, some of the smallest frogs in the world (Fig. 1). Using a variety of techniques, we have found that these animals cannot actually hear their own calls (Goutte et al., 2017). Wondering how else they might communicate, we discovered that the bones just under their skin fluoresce when illuminated with UV light (Goutte et al., 2019). We cannot yet say for sure whether these tiny toadlets can see the patterns so revealed though.
- Working with Prof. Pim Van Dijk and colleagues, I have created 3D models of the inner ears of several frogs. We discovered that the classic illustration of the frog ear created by E.G. Wever, which has been used in textbooks, lectures and articles for many years, is inaccurate in several respects. See Mason et al. (2015) for more.
- I have investigated ear function in the aquatic frog Xenopus laevis, best-known as an animal model in developmental biology studies. This strange frog lacks a tympanic membrane but instead has a cartilaginous tympanic disc, formed as an expansion of the extrastapes. The ear of this animal, like that of the bullfrog, is sexually dimorphic, with male Xenopus having much larger tympanic discs than females. A rocking movement of the stapes is found in the Xenopus ear, as in the bullfrog, but in Xenopus the extrastapes (tympanic disc) is more rigidly coupled to the stapes and the resulting lever ratio is much smaller (Mason et al., 2009). This probably represents an adaptation to improve hearing underwater.
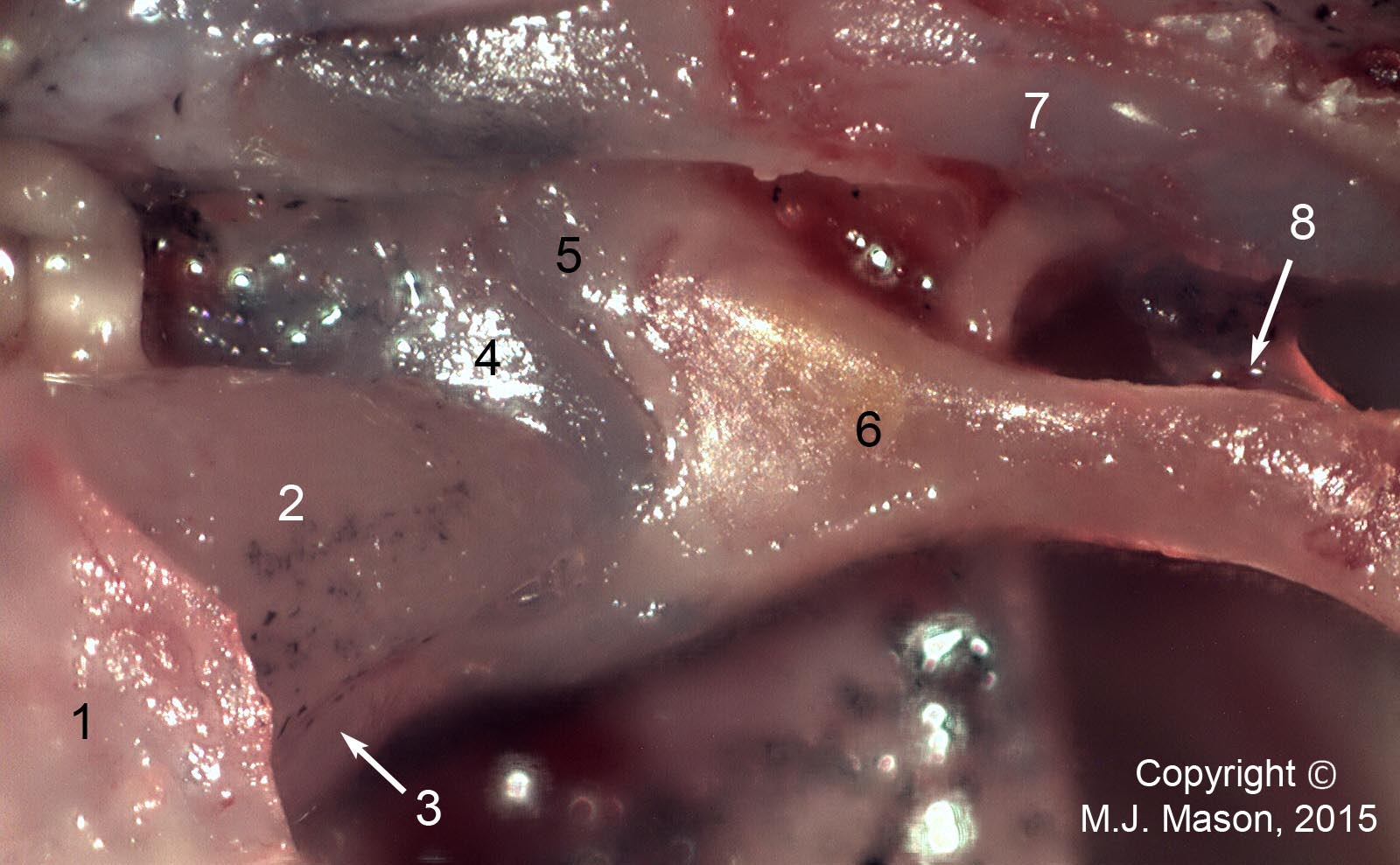
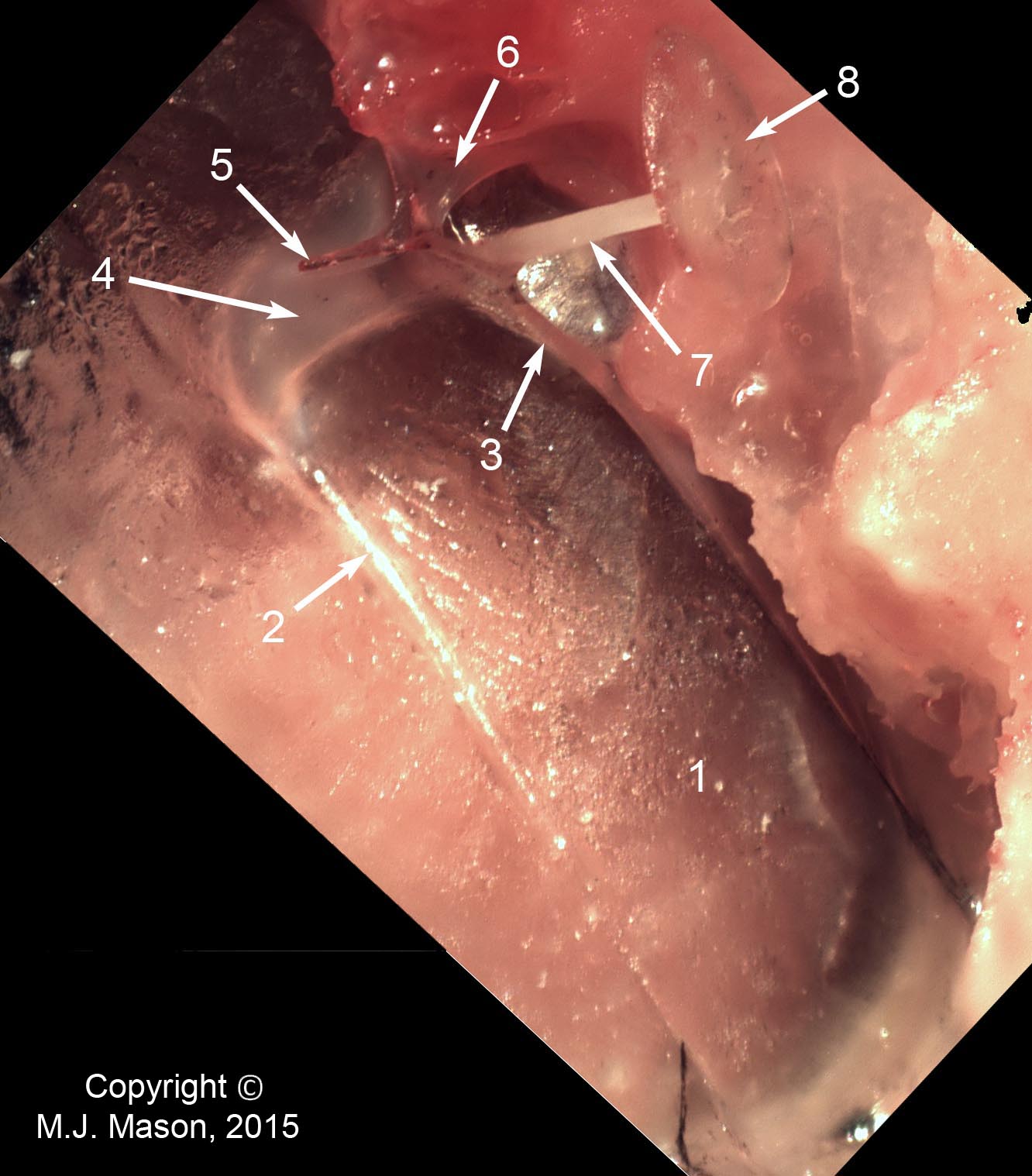
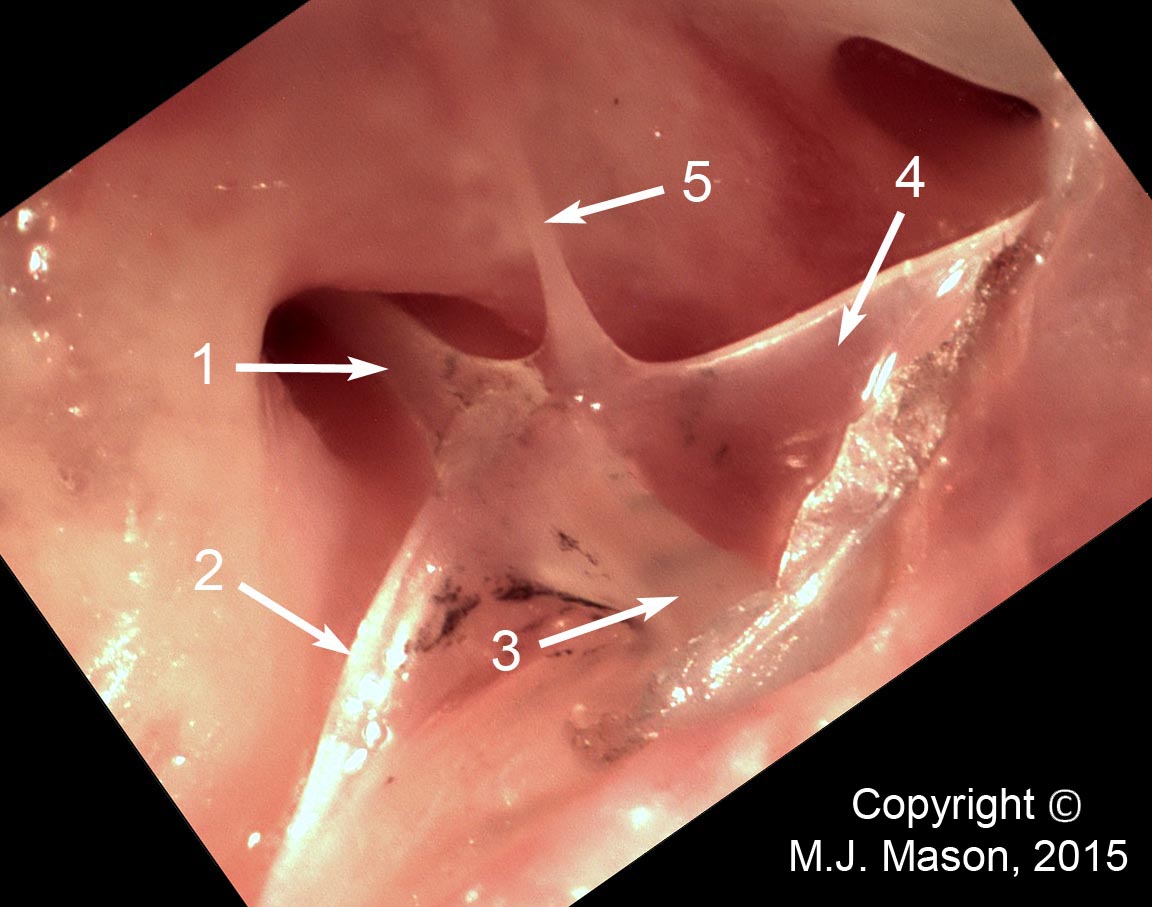
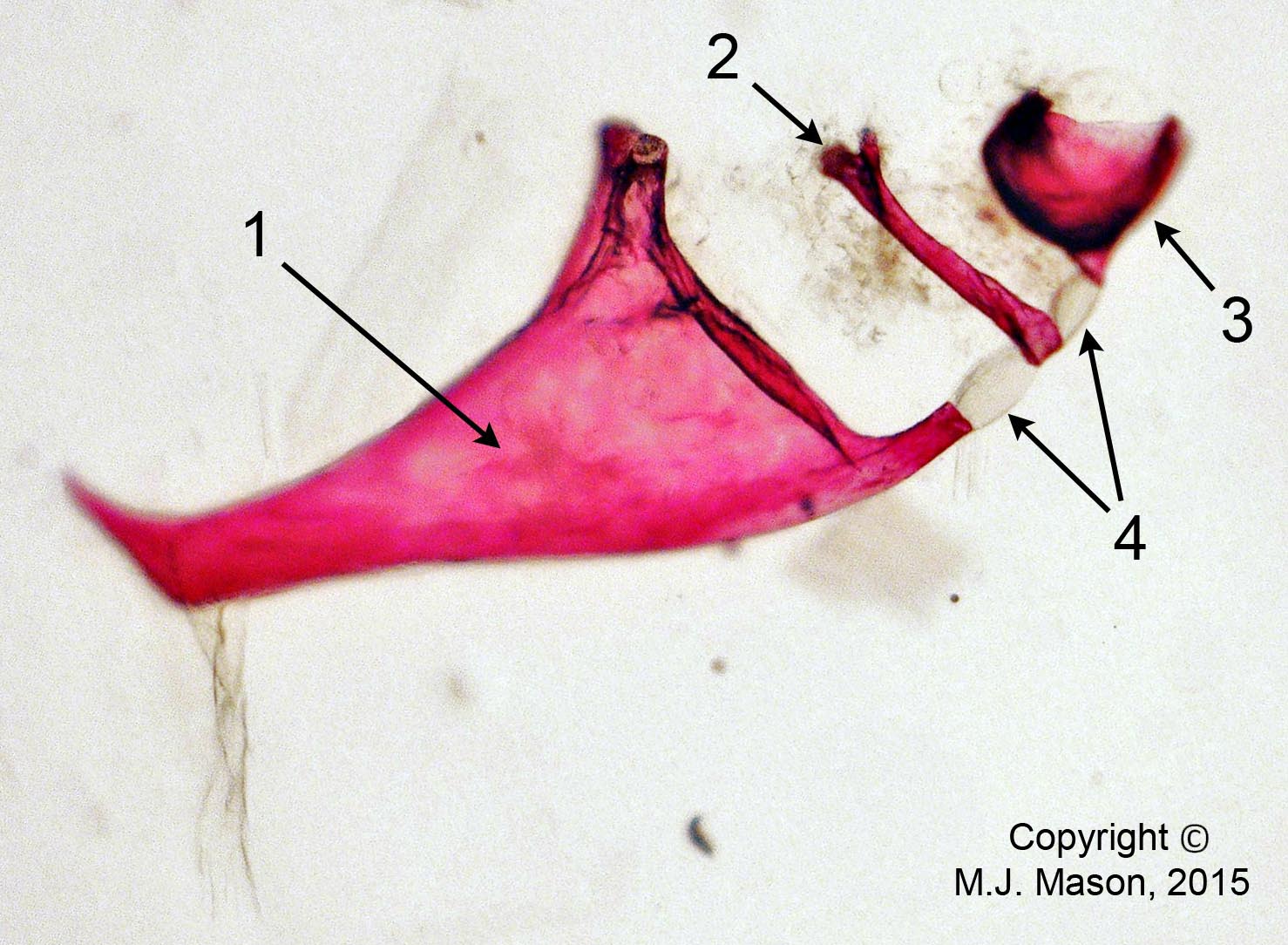
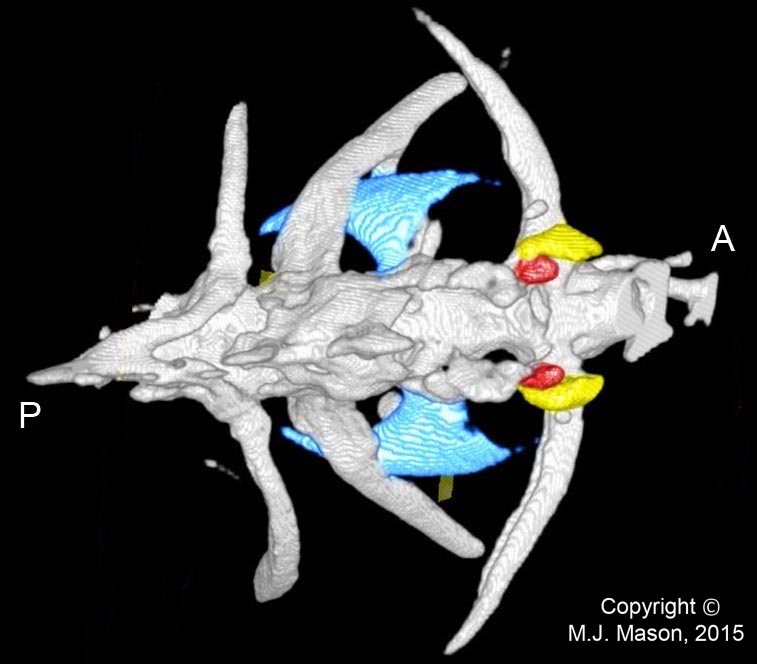
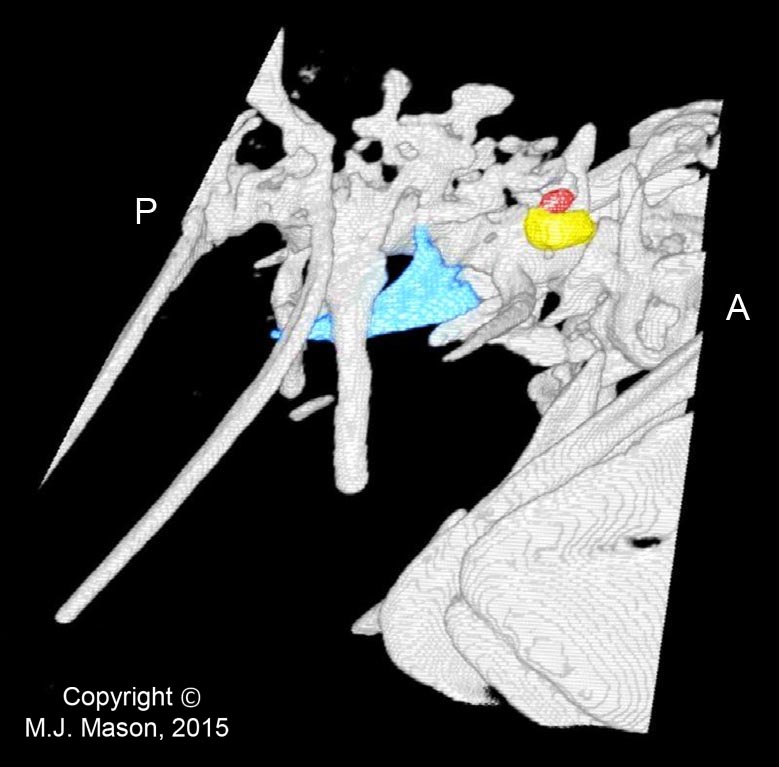
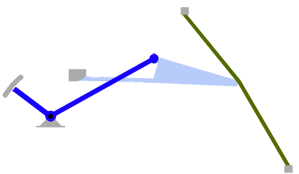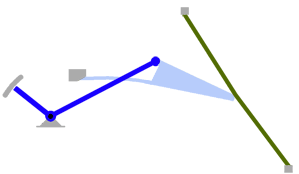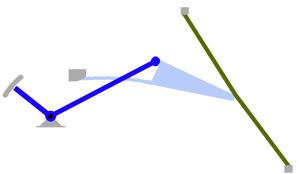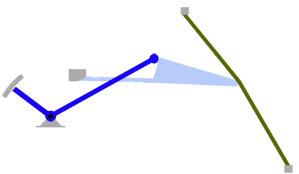
- Using a laser interferometer to make precise measurements of nanometer-scale movements, Prof. Peter Narins (of UCLA) and I were able to establish exactly how the middle ear apparatus vibrates in the bullfrog. We found that there is some flexibility between stapes and extrastapes (Fig. 2), and that a cartilaginous band called the ascending process of the extrastapes provides pivotal support. Although text-book illustrations tend to represent the stapes and extrastapes of frogs as simple pistons, the extrastapes of frogs actually works, in effect, as a second ossicle (Mason & Narins, 2002a)! Within the caudal half of the oval window in many frogs and salamanders is a second otic element called the operculum. Although long believed to be part of a separate pathway involved in seismic sensitivity, our laser interferometric work suggests that in bullfrogs the operculum is actually coupled to the stapes footplate: it moves in response to airborne sound (Mason & Narins, 2002b). The operculum may confer protection against quasi-static pressure changes associated with breathing and perhaps vocalization. Flexibility within the ossicular apparatus may turn out to be universal among terrestrial vertebrates, perhaps because of the advantages conferred with respect to pressure buffering. This is an intriguing avenue of research which I am continuing to explore (see Mason & Farr, 2013).
This work represents the results of a short undergraduate project performed in the Mason and Fleming laboratories by Rebecca Jordan in 2011.
Please contact Dr. Matthew Mason for more information about this as yet unpublished work.
With grateful thanks to Dr. Angeleen Fleming.