I am interested in the transduction and propagation of biological signaling events, particularly in the areas of skeletal and cardiac muscle activation, the modulation of osteoclast activity and spreading depression in the central nervous system, through a variety of electrophysiological, spectrofluometric, confocal/electronmicroscope, magnetic resonance imaging and mathematical modelling techniques.
(1) Activation of muscle through triggering of ryanodine receptor mediated release of Ca2+ stores
I demonstrated that the release of intracellularly stored Ca2+ that initiates muscle movement is triggered by conformational changes in regulatory intramembrane macromolecules following voltage change through proving a sequence of principles that: (1) small intramembrane electric currents or charge movements formed the electrical signature for such conformational changes. This led to (2) pharmacological resolution of their contributing component intramolecular species by Fourier Transform analyses of their charging signals and a demonstration that (3) each component constituted an independent membrane transition. I then implicated a specific (4) qγ, charging component whose whose voltage dependence, transverse tubular localization and pharmacological and nonlinear kinetic properties paralleled those of the observed intracellular Ca2+ signals.
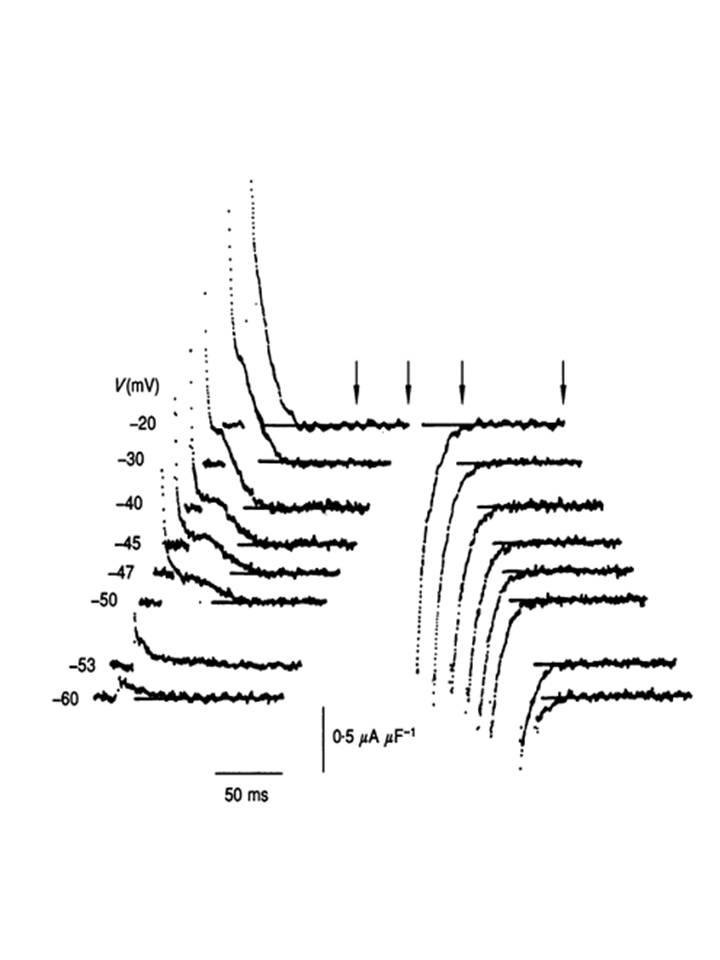
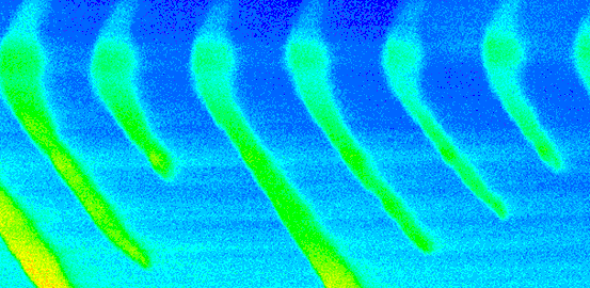
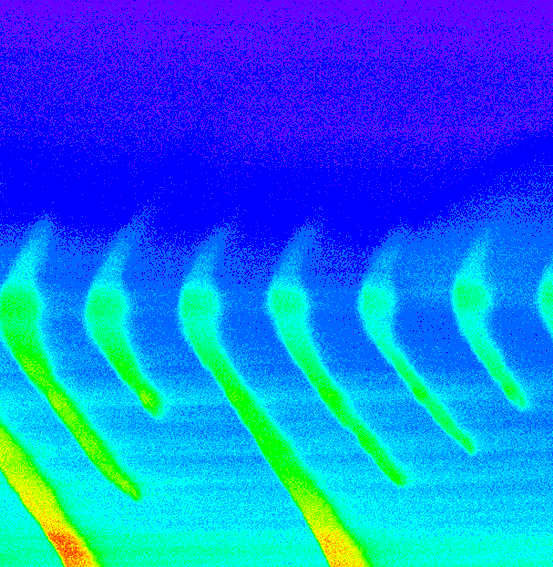
(5) Statistical mechanical and dielectric analysis demonstrated that this signal reflected a steeply voltage-dependent co-operative molecular process, that could be identified with (6) transitions within modified dihydropyridine receptors that showed an (7) allosteric engagement with RyR-Ca2+ release channels gating calcium release from intracellular stores. Thus, (8) depolarization drives a configurational change that dissociates this allosteric coupling and thereby frees the RyR to release stored Ca2+. This situation that can take place even in fully polarized and therefore otherwise quiescent fibres as exemplified in xt colour map plots of Ca2+ waves detected by fluo-3 fluorescence when the RyR was dissociated from its controlling dihydropyridine receptor-voltage sensor in the tubular membrane of skeletal muscle: front cover in the Journal of Physiology. This work is reviewed in the article:
http://link.springer.com/article/10.1007%2Fs10974-011-9262-9
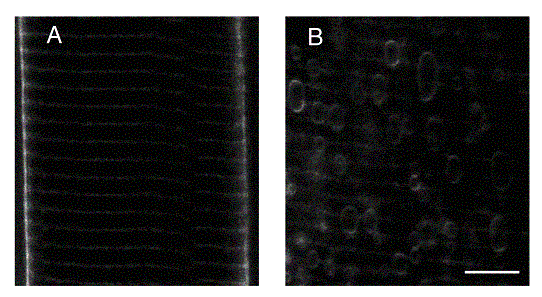
Studies of the function and structural stabilization of the transverse tubular system in which this transduction takes place revealed that (1) the tubular system selectively filters low-frequency components of the action potential signal to achieve the above activation. It leaves the high frequency components to propagate rapidly over the muscle surface membrane. However, (2) it appears to itself possess a capacity of regenerative electrical excitation as opposed to acting as a passive conductor of electrical activity in the surface membrane. (3) Repetitive activity can result in the vacuolation and tubular detachment also observed in pathological conditions such as muscular dystrophy, muscle fatigue, and osmotic stess and is exemplified for an intact fibre (left) and a vacuolated fibre (right) exemplified below. This led to development of (5) a quantitative model for the maintainence of transverse tubular integrity in which cellular water entry through a surface membrane Na-K-2Cl exchanger balanced water extrusion by Na-K-ATPase into the tubules.
This work is reviewed in the articles:
http://onlinelibrary.wiley.com/doi/10.1111/j.1469-185X.2008.00066.x/epdf
http://www.sciencedirect.com/science/article/pii/S0079610706001143
(2) The osteoclast as an exemplar for the activation of nonexcitable cells. Parallel mechanisms in nonexcitable cells also involving surface RyRs
A similar biophysical approach applied to single isolated osteoclasts, cells central to the pathogenesis of osteoporosis, successfully demonstrated, then characterized, parallel membrane controls of intracellular calcium redistribution in nonexcitable cells. These studies began by demonstrating that (1) osteoclasts operate at least two signaling pathways that involve different G-proteins and elevate either cytosolic [Ca2+] or [cAMP], whose (2) activation produces cell retraction or a quiescence of cell motility respectively. These findings led to demonstration of a local mechanism for control of the osteoclastic bone resorption cycle as shown in the sketch below. (3) an increased ambient calcium as might result from local hydroxyapatite dissolution elevated both cytosolic [Ca2+] and inhibited bone resorption. Microspectrofluorimetric studies of cellular responses to agonist applications then implicated (4) a novel, surface membrane 'calcium receptor' in this local functional regulation whose occupancy drove the calcium redistribution from intracellular stores and triggered a capacitative entry of external calcium. The latter reduced cell motility, adhesion upon substrate and therefore bone resorptive activity.
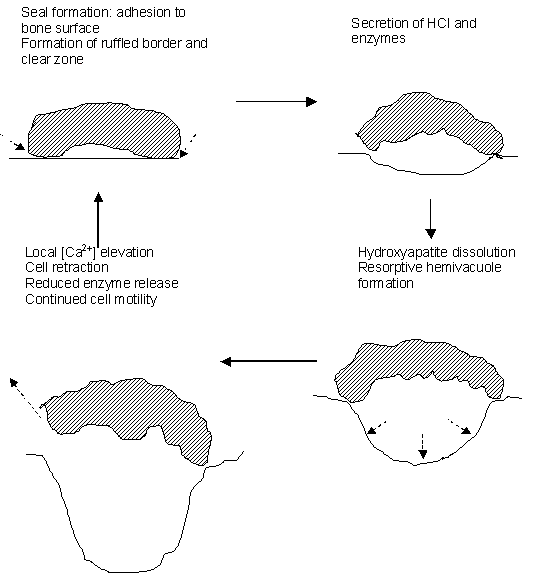
Confocal microscope studies that used specific fluorescent-labeled antibodies raised in muscle then demonstrated that (4) this local regulation of osteoclast activity involves a unique cell surface cardiac-muscle-type ryanodine receptor (RyR). This thus demonstrated both surface membrane RyRs and their involvement in osteoclast function for the first time. We then went on to demonstrate (5) similar operations of Ca2+ receptors in other cell systems, and have demonstrated (6) regulation of such osteoclast RyRs by caffeine, ryanodine itself, ruthenium red as well as cyclic ADP-ribose. (7) The most recent experiments have broadened the investigation of the role of RyRs in coupling signalling to metabolism in bone resorbing osteoclasts. This work is reviewed in:
http://onlinelibrary.wiley.com/doi/10.1017/S1464793103006262/epdf
http://www.sciencedirect.com/science/article/pii/S8756328202006889
http://onlinelibrary.wiley.com/doi/10.1111/j.1469-185X.2008.00048.x/epdf
(3) Tissue spread of cellular activation: cortical spreading depression
MRI is becoming increasingly promising as an investigative tool that will enable non-invasive studies of physiological processes at the whole organism level. Their development en passant led to (1) the first MRI demonstration of chronic cardiac muscle changes in vivo following spontaneous hypertension and experimental diabetes, but the primary objective was the development of (2) MRI methods to map strategic cerebral diffusion-related parameters to high resolution. The work dealt successfully with the theoretical, methodological and practical problems associated with development of robust optimized protocols for diffusion tensor measurements using as subject healthy human brain. This gave systematic high-resolution maps of the principal diffusivities and the associated rotationally invariant isotropy and anisotropy indices and a platform by which we could detect and analyze physiological abnormalities by such diffusion-weighted imaging (DWI).
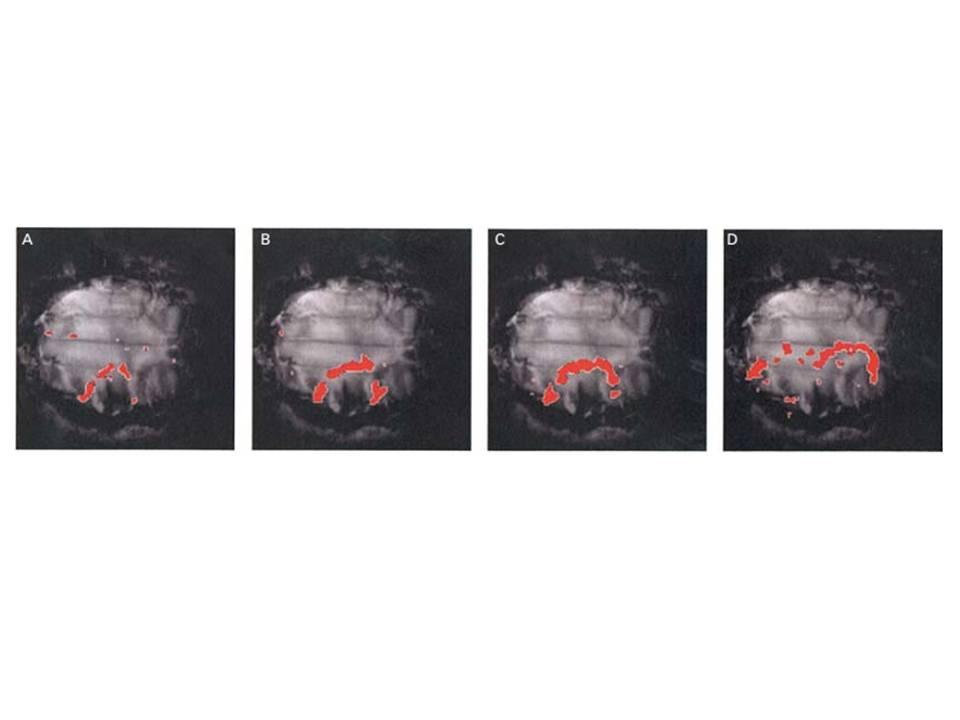
It was then possible to achieve (3) the first successful physiological demonstration and quantitative analysis of cortical spreading depression (CSD) evoked with KCl in the gyrencephalic brain, using time-lapse, diffusion-weighted MRI. CSD has been implicated in human migraine with aura and leads to a transient reduction in cerebral water diffusibility, which, hitherto, has been detected with DWI only in rodent brain. This made it possible to (4) characterize the spatial and temporal spread of the advancing ADC waves over the brain surface and explore (5) effects of the possible antimigraine agent, tonabersat upon CSD and of (6) hypercapnia, the antipsychotic agents sulpiride and mCPP, and (7) the Ca2+ antagonist MK-801 upon vascular changes in a similar mammalian brain preparation. This work is reviewed in:
http://onlinelibrary.wiley.com/doi/10.1111/j.1469-185X.2006.tb00214.x/epdf
(4) The electrical spread of cellular activation: translational studies on cardiac arrhythmogenesis.
Cardiac arrhythmias result from disruption of the electrical excitation processes that initiate cardiac contraction. Ventricular arrhythmias can lead to sudden cardiac death (SCD) which causes >300,000 and ~70,000 deaths/year in the USA and UK respectively. I am using transgenic models to clarify the mechanisms for arrhythmic disease, relating arrhythmic phenomena to electrophysiological properties that may be relevant to initiation and perpetuation of atrial or ventricular arrhythmias. My current work on cardiac arrhythmogenesis is exemplified by some of the illustrations in the margins of this website. This has resolved the roles of after-depolarization phenomena, conduction velocity, restitution gradients, refractoriness and altered intracellular Ca2+ homeostasis in ventricular arrhythmogenesis in hypokalaemic and genetically modified murine cardiac models for the Brugada, LQT3, LQT5 and catecholaminergic polymorphic ventricular tachycardic syndromes. Our work on the Brugada Syndrome won the 2014 research award from the British Society for Cardiac Research:
This work is reviewed in:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3308472/pdf/heartjnl-2011-300953.pdf

