Research
During embryogenesis, organs form by the transformation of simple epithelial layers of cells into complex 3D structures. This remarkable feat is accomplished through the collective and controlled changes in the shapes and arrangements of a large number of cells. The central aim of my group is to understand how genetic programmes drive morphologic changes in individual cells, and how those shape changes are coordinated through cell-cell interactions across an entire epithelium to sculpt a nascent tissue. Because organ shape is critical for organ function, defects in morphogenesis lead to severe diseases including spina bifida or polycystic kidney disease. Thus, understanding the mechanisms that drive faithful organ formation will elucidate a key aspect of metazoan development, help explain the pathogenesis of various developmental diseases, and may lead to new treatment opportunities.
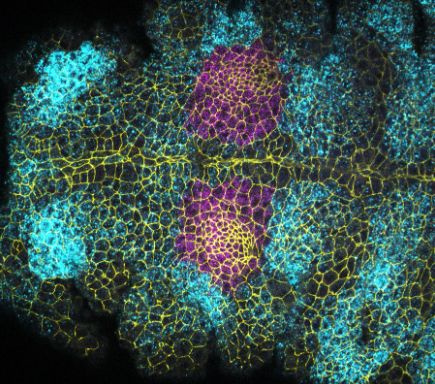
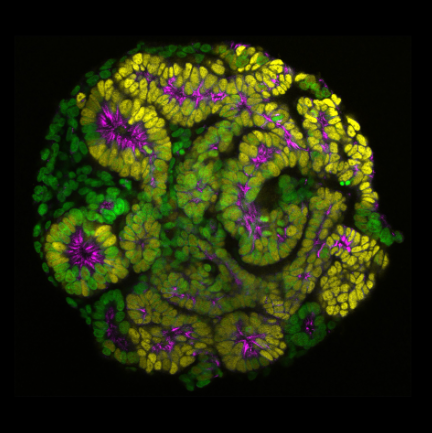
Many of the important organ systems in mammals and invertebrates are tubular in structure, including the intestinal tract, kidney, liver, lung, vasculature, and most glandular organs. Tube formation is thus a critical step in organogenesis, and can occur through a number of mechanisms that include the folding, wrapping, or budding of epithelial sheets. My lab investigates two models of tube morphogenesis. Our major focus has been the salivary glands of the Drosophila embryo, with more recent second focus on the formation of the nephron tube in human renal organoids in culture.
Publications
Gillard, G. and Röper, K (2024). b-H-Spectrin is a key component of an apical-medial hub of proteins during cell wedging in tube morphogenesis. J. Cell Science 137 (15): jcs261946
Ng-Blichfeldt, J.-P., Stewart, B.J., Clatworthy, M.R., Williams, J.M., and Röper, K. (2024) Identification of a core transcriptional program driving the human renal mesenchymal-to-epithelial transition. Developmental Cell (59), 1–18, https://doi.org/10.1016/j.devcel.2024.01.011
Peterson, J., Balogh Sivars, K. Bianco, A., and Röper, K. (2023.) Toll-like receptor signalling via IRAK4 affects epithelial integrity and tightness through regulation of junctional tension. Development 150 (24): dev201893;
Ashour, D.J., Durney, C.H., Planelles-Herrero, V.J., Stevens, T.J., Feng,J.J. and Röper, K. (2023) Zasp52 strengthens whole embryo tissue integrity through supracellular actomyosin networks. Development 150, dev20123
Sánchez-Corrales, Y. E., Blanchard, G. B., & Röper, K. (2021) Correct regionalisation of a tissue primordium is essential for coordinated morphogenesis. eLife; 10:e72369. https://doi.org/10.7554/eLife.72369
Gillard, G., Girdler, G. and Röper, K. (2021). A release-and-capture mechanism generates an essential non-centrosomal microtubule array during tube budding. Nature Communications 12: 4096. https://doi.org/10.1038/s41467-021-24332-0
Sidor, C., Stevens, T., Jin, L., Boulanger, J., and Röper, K. (2020). Rho kinase planar polarisation at tissue boundaries depends on phospho-regulation of membrane residence time. Developmental Cell, 52: 364–378. https://doi.org/10.1016/j.devcel.2019.12.003
Sánchez-Corrales, Y. E., Blanchard, G. B., and Röper, K. (2018). Radially-patterned cell behaviours during tube budding from an epithelium. eLife, 7: e35717. http://doi.org/10.7554/eLife.35717/