Submitted by Emily Rigby on Fri, 07/06/2024 - 14:07
The extension of the ectoderm in gastrulating fruit fly embryos is remarkably robust to a pull in the perpendicular direction caused by the invagination of the neighbouring mesoderm.
Embryonic development is both fascinating and visually dramatic. As the embryo takes shape, cells generate forces and tissues undergo substantial movements, and in doing so pull and push on neighbouring tissues. Since these neighbouring tissues are made up of mechanically sensitive cells, we need to ask ourselves ‘to what extent are these pushes and pulls important for development: do they help, or do they hinder?’
An increasing number of examples have been reported where such pulls and pushes help the morphogenesis of the neighbouring tissues, but is this always the case? Claire Lye, Guy Blanchard, Jenny Evans, Alexander Nestor-Bergmann and Bénédicte Sanson have found that it is not, in their recent study “Polarised cell intercalation during Drosophila axis extension is robust to an orthogonal pull by the invaginating mesoderm” published in PLOS Biology.
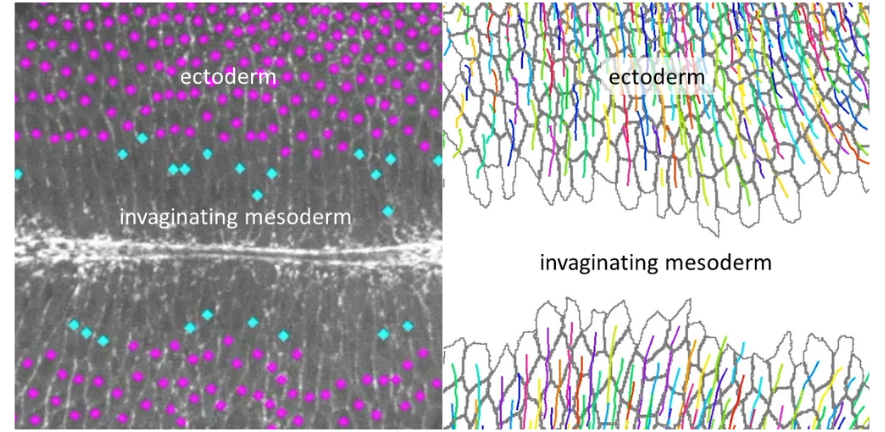
Gastrulation in Drosophila has become a valued model for the study of the impact of extrinsic forces on the morphogenesis of developing tissues, as three major tissue movements occur simultaneously: the mesoderm invaginates ventrally, the endoderm invaginates posteriorly and convergence and extension of the ectoderm (germband extension) begins. While it has been previously demonstrated that the endoderm mechanically aids the extension of the germband by causing a parallel tensile force from the posterior, the impact of the mesoderm on germband extension has been unclear.
Germband extension is driven primarily by polarised cell intercalation downstream of genetic patterning; cell junctions between anterior and posterior cell neighbours shrink due to the polarised localisation of Myosin II, which contracts the actin cortex, and a new cell junction then grows in the perpendicular direction. This causes cells to rearrange their location with respect to one another, causing the body axis to extend in the anterior-posterior direction and narrow in the dorso-ventral (DV) direction.
Because Myosin II can be recruited to sites of mechanical tension, it had been proposed that a ventral pull from the invaginating mesoderm might augment the polarised localisation of Myosin II to DV-oriented shrinking cell junctions, thus speeding up the shrinkage of cell junctions during cell intercalation and therefore germband extension itself. An alternative hypothesis is that mesoderm invagination might pull on the DV-oriented junctions making it harder for them to shrink, and potentially stretching them causing a dilution of Myosin II, causing germband extension to slow down.
Claire Lye and colleagues acquired comparable live-imaging datasets of the germband of normal Drosophila embryos (wildtype) and those lacking mesoderm invagination (twist mutants) and analysed this data to quantify the behaviour of the tissue, cells and cell junctions. They found no significant positive or negative effect of mesoderm invagination on the amount of Myosin II at DV-oriented junctions, the speed of junction shrinkage or the rate of cell intercalation.
Therefore, the pull from the invagination of the mesoderm does not help to speed up polarised cell intercalation through mechanotransduction, nor does it slow it down through mechanical effects. Moreover, although the density of Myosin II at DV-oriented junctions is not increased in response to mesoderm invagination, it is also not diluted despite significant stretching of these junctions. This study reveals that a homeostatic mechanism must exist to maintain Myosin II at an appropriate concentration at these junctions to drive cell intercalation at the correct speed. The Sanson lab found that polarised cell intercalation is remarkably robust to the extrinsic force exerted by the invaginating mesoderm.
This shows that mechanical interactions between tissues do not always elicit a significant response: tissue context is important in governing the response of a tissue to an extrinsic force generated by a neighbouring tissue. Dr Claire Lye says “If we want to use our knowledge of developmental biology to drive future breakthroughs in tissue engineering, we will need to understand the range of responses a tissue can have in response to an extrinsic force, and exactly how this is controlled”.
Despite the robustness of the speed of cell intercalation, the authors did find some other changes in the behaviour of cells and cell junctions during germband extension in response to mesoderm invagination. To find out more, and for further discussion of the tissue context underlying the robustness of cell intercalation during germband extension, read the full paper: Polarised cell intercalation during Drosophila axis extension is robust to an orthogonal pull by the invaginating mesoderm | PLOS Biology

