Biography
Christine Holt received a BSc Hons degree in Biological Sciences from the University of Sussex in 1977 and a PhD degree in Zoology from King’s College, London University in 1982. She did her postdoctoral training in the Physiology Department at Oxford University where she was also a Junior Research Fellow at Worcester College, and in the Biology Department at the University of California San Diego (UCSD). In 1992, she joined the faculty at UCSD and became a tenured Associate Professor in 1996. In 1997, she moved to the University of Cambridge as a Lecturer in the Anatomy Department and a Fellow of Gonville and Caius College. In 2003 she became the Professor of Developmental Neuroscience in the Department of Physiology, Development and Neuroscience. She was a Pew Scholar and a McKnight Scholar in her early career and has been the recipient of numerous grant awards from the NIH, MRC, HFSP and Wellcome Trust and ERC Advanced Grant. She has served on various Scientific Advisory Boards, Editorial Boards and Selection Committees. In recognition of her research, she was elected a Member of EMBO (2006), Fellow of the Medical Academy of Sciences (2007), Fellow of The Royal Society (2009), Fellow of the Royal Society of Biology (2011), Honorary Fellow of Worcester College, Oxford (2019) and International Member of the National Academy of Sciences, USA (2020), Honorary Member of the Physiological Society (2021). She has been awarded several international prizes: the Remedios Caro Almela Prize for Research in Developmental Neurobiology (2011), the Champalimaud Vision Award (2016) and the Royal Society Ferrier Medal (2017), the Rosenstiel Award (2022), and the Brain Prize (2023).
Christine Holt became Professor Emerita in 2019. She remains actively engaged in science-related endeavours. She is not accepting applications for summer internships or research studentships.
Research
Wiring the brain: RNA-based mechanisms of axon guidance and maintenance
Christine Holt is a Developmental Neuroscientist who studies how nerve connections are formed and maintained in the brain. In the vertebrate visual system, neurons in the eye extend axons over long distances to find their synaptic targets in the brain. This impressive navigational feat, which occurs during embryonic development (before birth in humans), underlies the precise wiring of the mature brain and is essential for building functional nerve connections. The goal of her research is to understand the molecular and cellular mechanisms that guide and maintain axons. She uses multidisciplinary approaches such as in vivo gene transfer, growth cone chemotropic assays, time-lapse and single molecule imaging and genome-wide analysis in vertebrate model systems
(Xenopus, zebrafish and mouse). She has focused on particular ‘steering points’ within the visual pathway where axons alter their direction of growth, such as the optic chiasm and the optic nerve head. Her group developed one of the first in vivo gene transfer/expression approaches (1990) and found that ephrin-B is important in regulating the divergent routing of axons at the chiasm and in retinotectal map formation. Her group also identified molecular pathways important for axon growth and target entry and showed that netrin-1/DCC/laminin-1 interactions play a key role in directing axons out of the eye. In 2001, her group discovered that the growing tips of axons, the growth cones, rapidly synthesize and degrade new proteins in response to guidance cues and that inhibition of axonal protein synthesis, or degradation, blocks directional guidance. Since then her studies have focused largely on local translation and mRNA localization in axons. Her group used laser-capture technology to reveal that hundreds of mRNAs reside in axons and growth cones and went on to show that the localised synthesis of specific proteins, such as beta-actin, is required for axon turning and branching. Her group developed a subcellular genome-wide approach to investigate the mRNAs translated in axon terminals in vivo (Axon-TRAP-RNAseq) and showed that thousands of mRNAs are translated in both growing and mature retinal axons. Her group also provided some of the first evidence to show that axons are kept alive in vivo by the local synthesis of new proteins that help to sustain mitochondrial function (2012), opening new approaches to neurodegeneration. Her recent work provides novel evidence that ribosomes can be modified in axons by incorporating newly synthesized ribosomal proteins suggesting the possibility that ribosomes can be repaired locally, and possibly ‘tuned’ for optimal translation. By studying the cell biology of axons her work aims to gain a better understanding of how nerve connections are first established and how they are sustained throughout the lifetime of an animal. Fundamental knowledge of this sort is essential for understanding neurodevelopmental and neurodegenerative disorders and for developing clinical therapies in nerve repair.
Main sources of research funding: Wellcome Trust, ERC, Champalimaud Foundation Award.
Her Royal Society Ferrier Medal Prize Lecture (a Public Lecture) can be viewed at: https://royalsociety.org/science-events-and-lectures/2017/03/ferrier-lecture/
Collaborator/ colleagues
Bill Harris (PDN, Cambridge)
Julie Qiaojin Lin (Guangzhou University, China)
Clemens Kaminski (Department of Chemical Engineering and Biotechnology, Cambridge)
Hosung Jung (Yonsei University College of Medicine, Seoul)
Nicholas Clifton (University of Exeter Medical School)
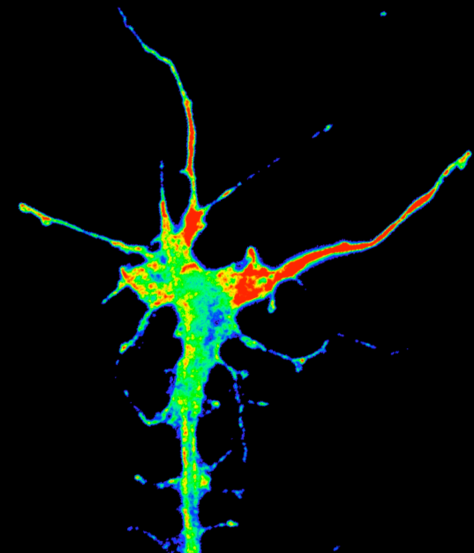
Translation of beta-actin in axon terminal in brain.
Timelapse movie of RNA granules (white) and new beta-actin protein (coloured) in a retinal axon terminal in the brain of Xenopus. New protein appears to be 'painted' by mRNA granules where translation is occurring. (Hovy Wong).
Publications
Holt CE. Biological Roles of Local Protein Synthesis in Axons: A Journey of Discovery. Annu Rev Genet. 2024 Nov;58(1):1-18. doi: 10.1146/annurev-genet-072220-030822. Epub 2024 Nov 14.PMID: 39121543. Review.
van Tartwijk FW, Wunderlich LCS, Mela I, Makarchuk S, Jakobs MAH, Qamar S, Franze K, Kaminski Schierle GS, St George-Hyslop PH, Lin JQ, Holt CE, Kaminski CF. Mutation of the ALS-/FTD-Associated RNA-Binding Protein FUS Affects Axonal Development. J Neurosci. 2024 Jul 3;44(27):e2148232024. doi: 10.1523/JNEUROSCI.2148-23.2024.PMID: 38692734.
Clifton NE, Lin JQ, Holt CE, O'Donovan MC, Mill J. Enrichment of the Local Synaptic Translatome for Genetic Risk Associated With Schizophrenia and Autism Spectrum Disorder. Biol Psychiatry. 2024 May 1;95(9):888-895. doi: 10.1016/j.biopsych.2023.12.006. Epub 2023 Dec 14.PMID: 38103876
Jung J, Ohk J, Kim H, Holt CE, Park HJ, Jung H. mRNA transport, translation, and decay in adult mammalian central nervous system axons. Neuron. 2023 Mar 1;111(5):650-668.e4. doi: 10.1016/j.neuron.2022.12.015. Epub 2022 Dec 29.PMID: 36584679
Koppers M, Holt CE. Receptor-Ribosome Coupling: A Link Between Extrinsic Signals and mRNA Translation in Neuronal Compartments. Annu Rev Neurosci. 2022 Jan 5. doi: 10.1146/annurev-neuro-083021-110015. PMID: 34985917
Lin JQ, van Tartwijk FW, Holt CE Axonal mRNA translation in neurological disorders. RNA Biol. 2021 Jul;18(7):936-961. doi: 10.1080/15476286.2020.1822638. Epub 2020 Sep 29. PMID: 32988274
Lu M, van Tartwijk FW, Lin JQ, Nijenhuis W, Parutto P, Fantham M, Christensen CN, Avezov E, Holt CE, Tunnacliffe A, Holcman D, Kapitein L, Schierle GSK, Kaminski CF. The structure and global distribution of the endoplasmic reticulum network are actively regulated by lysosomes. Sci Adv. 2020 Dec 16;6(51):eabc7209. PMID: 33328230
Farias J, Holt CE, Sotelo JR, Sotelo-Silveira JR. Axon microdissection and transcriptome profiling reveals the in vivo RNA content of fully differentiated myelinated motor axons. RNA. 2020 May;26(5):595-612. doi: 10.1261/rna.073700.119.
Shigeoka T, Koppers M, Wong HH, Lin JQ, Cagnetta R, Dwivedy A, de Freitas Nascimento J, van Tartwijk FW, Ströhl F, Cioni JM, Schaeffer J, Carrington M, Kaminski CF, Jung H, Harris WA, Holt CE. On-Site Ribosome Remodeling by Locally Synthesized Ribosomal Proteins in Axons. Cell Rep. 2019 Dec 10;29(11):3605-3619.e10. doi: 10.1016/j.celrep.2019.11.025.
Koppers M, Cagnetta R, Shigeoka T, Wunderlich LC, Vallejo-Ramirez P, Qiaojin Lin J, Zhao S, Jakobs MA, Dwivedy A, Minett MS, Bellon A, Kaminski CF, Harris WA, Flanagan JG, Holt CE. Receptor-specific interactome as a hub for rapid cue-induced selective translation in axons. Elife. 2019 Nov 20;8:e48718. doi: 10.7554/eLife.48718.
Holt CE, Martin KC, Schuman EM. Local translation in neurons: visualization and function. Nat Struct Mol Biol. 2019 Jul;26(7):557-566. doi: 10.1038/s41594-019-0263-5. Epub 2019 Jul 3.PMID: 31270476 Review.
Cioni, J-M, JQ Lin, AV Holtermann, M Koppers, MAH Jakobs, A Azizi, B Turner-Bridger, T Shigeoka, K Franze, WA Harris and CE Holt. Late endosomes serve as platforms for mRNA translation in axons and promote mitochondrial and axonal integrity. Cell 2019 Jan 10;176(1-2):56-72.e15. Epub 2019 Jan 3. Highlighted in Spotlight Neuron 101, January 16, 2019.
Thompson AJ, Pillai EK, Dimov IB, Foster SK, Holt CE, Franze K. Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. Elife. 2019 Jan 15;8. pii: e39356.
Cagnetta, Roberta, Hovy Ho-Wai Wong, Christian K. Frese, Giovanna R. Mallucci, Jeroen Krijgsveld, Christine E. Holt. Noncanonical modulation of eIF2 pathway controls increase in local translation during neural wiring. Molecular Cell 2018 Dec 4. pii: S1097-2765(18)30983-3. [Epub ahead of print]
Turner-Bridger B, Jakobs M, Muresan L, Wong HH, Franze K, Harris WA, Holt CE. Single-molecule analysis of endogenous β-actin mRNA trafficking reveals a mechanism for compartmentalized mRNA localization in axons. Proc Natl Acad Sci U S A. 2018 Sep 25. pii: 201806189.
Leung, K-M, Bo Lu, Hovy Ho-Wai Wong, Benita Turner-Bridger, Christine E. Holt. Cue-polarized transport of β-actin mRNA depends on 3' UTR and microtubules in live growth cones. Frontiers in Cellular Neuroscience 2018 Sep 10;12:300. doi: 10.3389/fncel.2018.00300.
Cagnetta R, Frese CK, Shigeoka T, Krijgsveld J, Holt CE. Rapid Cue-Specific Remodeling of the Nascent Axonal Proteome. Neuron. 2018 Jul 11;99(1):29-46.e4. Epub 2018 Jun 28.
Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, Phillips EC, Miyashita A, Williams D, Ströhl F, Meadows W, Ferry R, Dardov VJ, Tartaglia GG, Farrer LA, Kaminski Schierle GS, Kaminski CF, Holt CE, Fraser PE, Schmitt-Ulms G, Klenerman D, Knowles T, Vendruscolo M, St George-Hyslop P. FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell. 2018 Apr 19;173(3):720-734.e15.
Cioni JM, Koppers M, Holt CE. Molecular control of local translation in axon development and maintenance. Curr Opin Neurobiol. 2018 Aug;51:86-94. Epub 2018 Mar 14. Review.
Cioni JM, Wong HH, Bressan D, Kodama L, Harris WA, Holt CE. Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron. 2018 Mar 7;97(5):1078-1093.e6.
Wong HH, Lin JQ, Ströhl F, Roque CG, Cioni, JM, Cagnetta R, Turner-Bridger B, Laine R, Harris WA, Kaminski CF, Holt CE. RNA docking and local translation regulate site-specific axon remodelling in vivo. Neuron 95(4) Aug (2017).
Ströhl, F, Lin, QJ, Laine, RF, Wong, HH, Urbancic,V, Cagnetta, R, Holt, CE, Kaminski, CF, (2017), Single Molecule Translation Imaging Visualizes the Dynamics of Local β-Actin Synthesis in Retinal Axons, Scientific Reports7(1):709 06 Apr
Shigeoka T, Jung H, Jung J, Turner-Bridger, B, Ohk J, Lin JQ, Amieux PS, Holt CE, (2016), Dynamic axonal translation in developing and mature visual circuits, Cell, pii: S0092-8674(16)30580-3. [Epub ahead of print]
Murakami T, Qamar S, Lin JQ, (et al 23 authors), Holt CE, Vendruscolo M, Kaminski CF, St George-Hyslop P, (2015),ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function, Neuron 18;88(4):678-90
Jung H, Gkogkas CG, Sonenberg N, Holt CE, (2014), Remote control of gene function by local translation, Cell 27;157 (1):26-40. Invited Review
Holt CE, Schuman EM, (2013), The central dogma decentralized: new perspectives on RNA function and local translation in neurons, Neuron, 80(3):648-57. Invited review
Leung LC, Urbancic V, Baudet ML, Dwivedy A, Bayley TG, Lee AC, Harris WA, Holt CE, (2013), Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation, Nature Neurosci, 16:166-73.
Jung H, Yoon BC, Holt CE, (2012), Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair, Nature Reviews Neuroscience, 13:308-24
Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE, (2012), Local translation of extranuclear lamin B promotes axon maintenance, Cell, 148:752-64
Baudet, M-L, Zivraj K, Abreu-Goodger C, Muldal A, Armisen J, Blenkiron C, Goldstein LD, Miska EA, Holt CE, (2011), miR-124 acts via CoREST to control onset of Sema3A sensitivity in navigating retinal growth cones, Nature Neuroscience, 15:2-38
Zivraj K, Tung L, Piper M, Gumy L, Fawcett J, Yeo G, Holt CE, (2010), Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs, J Neurosci 30:15464-78
Drinjakovic J, Jung H, Campbell DS, Strochlic L, Holt CE, (2010), E3 ligase Nedd4 promotes axon branching by down-regulating PTEN, Neuron, 65:341-57
Holt CE, Bullock S, (2009), Subcellular mRNA Localization in Animal Cells and Why it Matters, Science, 326:1212-6
Leung K-M, van Horck FPG, Andrew C Lin, Allison R, Standart N, Holt CE, (2006), Asymmetrical b-actin mRNA translation in growth cones mediates attractive turning to netrin-1, Nature Neuroscience, 9:1247-56
Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung K-M, Cogill E, Holt C, (2006), Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones, Neuron, 49:215-28
Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris WA, Prochiantz A, Holt CE, (2005), The transcription factor Engrailed-2 guides retinal axons, Nature, 438:94-98
Piper M, Salih S, Weinl C, Holt CE, Harris WA, (2005), Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation, Nature Neuroscience, 8:179-86
Shewan DS, Dwivedy A, Anderson R, Holt CE, (2002), Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway, Nature Neuroscience, 5:955-62
Mann F, Ray S, Harris WA, Holt CE, (2002), Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signalling through ephrin-B ligands, Neuron, 35:461-73
Campbell DS, Holt CE, (2001), Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation, Neuron, 32:1013-26
Nakagawa S-I, Brennan C, Johnson K, Shewan D, Harris WA, Holt CE, (2000), Ephrin-B regulates the ipsilateral routing of retinal axons at the optic chiasm, Neuron, 25:599-610
Hoepker VH, Shewan D, Tessier-Lavigne M, Poo M-M, Holt CE, (1999), Growth cone attraction to netrin-1 is converted to repulsion by laminin-1, Nature, 401:69-73